Abstract
Background: Steroid resistant (SR) acute Graft versus Host Disease (aGvHD) is a life-threating complication of allogeneic- stem cell transplantation (SCT); outcomes in grade III-IV are poor with mortality rates as high as 90%. Vedolizumab (Vd) is a monoclonal antibody inhibiting the binding of integrin α4β7 to Mad CAM -1, consequently impairing homing of T cells to the gastrointestinal (GI) endothelium. It is an approved therapy for inflammatory bowel disease (IBD). Encouraging results were recently reported by Floisand et al., in 6 patients (pts) with SR GI aGvHD (BBMT, 2017). We report a single center experience with the use of Vd in pts with severe SR GI GvHD.
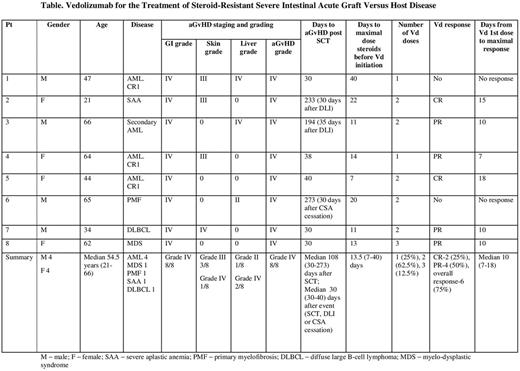
Methods: Medical records of 8 pts treated with Vd for SR GI aGvHD were reviewed. SR GI aGvHD was defined as progressive or refractory disease after receiving cyclosporine (CSA) and methylprednisolone (MP) at a dose of 2 mg/kg. Vd was administered at a dose of 300 mg IV, with planned repeated infusion after 2 and 6 weeks. Complete response (CR) was defined as complete resolution of GI aGvHD symptoms , including stopping of analgesics, total parenteral nutrition (TPN), and steroids. Partial response (PR) was defined as improvement in GI GvHD from grade III-IV to grade I-II.
Results: Overall, 8 pts were included with a median age of 54 years (range, 21-66). AML was the leading diagnosis (50%). Graft source was peripheral blood in all patients. The majority of pts had HLA matched unrelated donor (5), followed by matched sibling donor (2), and a mismatched unrelated donor (1). Most pts received myeloablative conditioning regimens (6); GvHD prophylaxis included CSA (8) and Methotrexate (MTX) (6) or Mycophenolate Mofetil (MMF) (2). Anti Thyomoctye Globulin (ATG) was given to 7 pts.
All pts were diagnosed with GI aGvHD according to clinical manifestations. Colonic biopsies, supporting the diagnosis, were performed in 4 pts. Liver and skin aGvHD was noted in 3 and 4 pts, respectively. Prior to Vd all patients had received IV MP 2mg/kg/day, CSA, oral non-absorbable steroids and MMF. TPN was given to 7 pts. Two patients had developed posterior reversible encephalopathy syndrome and were put off CSA (1 before and 1 after Vd).
Five pts developed aGvHD at median of 30 days post SCT, 2 pts at 30 days post-donor lymphocyte infusion (DLI) and 1 pt at 9 months post SCT (30 days following CSA cessation). The majority of pts received only 2 of the 3 doses of Vd (5/8 pts). Two pts experienced multi-organ failure prior to Vd initiation, and died soon after the intervention. Six pts had a clinical GvHD response within a median of 10 days (range, 7-24 days) from Vd first administration. Two pts had CR and 4 PR of the GI aGvHD. Two CR pts were able to be taken off immunosuppression (Table). However, 3/6 pts succumbed to infection complications at median of 7.5 months (range, 2-10 months) post transplantation. Of the two patients in achieving CR of aGvHD, one developed severe neurologic dysfunction with features of Guillain-Barré syndrome and the other succumbed to AML relapse. Following Vd, 5 patients had a CMV reactivation, although 4 had experienced prior reactivations. No other viral infections (EBV, HHV6, HSV, JCV) were observed. Three pts had probable invasive aspergillosis infection and 7 patients had signs consistent with bacterial infections with or without bacteremia.
Conclusions: Our data suggest that targeting integrin a4b7 can ameliorate severe SR GI aGvHD. Whether the high infection rates observed following administration are related to Vd or to prolonged immunosuppression is inconclusive. The timing, role and safety of Vedolizumab administration should be further explored in a clinical trial setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal